Introduction: VRD (bortezomib, lenalidomide, dexamethasone) and VCD (bortezomib, cyclophosphamide, dexamethasone) are the two most commonly used triplet induction regimens in transplant eligible patients with multiple myeloma (MM). VCD is commonly used in patients with renal dysfunction at diagnosis. Given the variable results seen in head to head comparison of these (or similar) regimens to date, no definitive conclusion can be made on whether they are equivalent. This large CIBMTR registry study compares the outcomes with VRD vs. VCD induction therapy in patients undergoing upfront autologous stem cell transplant (ASCT).
Methods: Patients were included if they underwent upfront ASCT for MM from January 2013 to December 2018 within 6 months of diagnosis, received VRD or VCD induction therapy and achieved at least a partial response pre-transplant. Of 1,135 patients, 914 patients received VRD and 221 received VCD therapy. Cox proportional hazards models were created for progression-free survival (PFS) and overall survival (OS). The following covariates were adjusted in the models: induction regimen (main effect), age, sex, race, performance score, hematopoietic cell transplant-comorbidity index (HCT-CI), estimated glomerular filtration rate (eGFR) at diagnosis, immunoglobulin subtype, cytogenetics, ISS stage, response status at transplant, melphalan dose, and maintenance therapy.
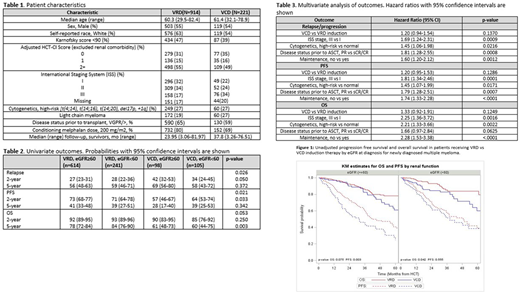
Results: As shown in Table 1, patients in the VRD and VCD cohorts had similar age at transplant, adjusted HCT-CI scores, and distribution of high-risk cytogenetics. Patients in the VCD group were more likely to have renal impairment and ISS stage III disease. An eGFR (mL/min/1.73m2) at diagnosis < 60 was seen in 26% of VRD patients vs. 48% of VCD patients. ISS stage III was seen in 17% vs. 34% of patients, respectively.
Treatment and Response: Pre-transplant response in the VRD cohort was: complete response (CR)- 17%, very good partial response (VGPR)- 48% and partial response (PR)- 35%. Response in the VCD cohort was: CR- 17%, VGPR- 42% and PR- 41%. Conditioning dose was melphalan 200 mg/m2 in 80% of patients receiving VRD vs. 69% in the VCD group. Post-transplant response was at least VGPR in 74% vs. 75% of patients, respectively. Maintenance in the VRD group was: lenalidomide based (+/- bortezomib)- 80%, bortezomib based- 5%, other-3% and none- 12% of patients. In the VCD group, maintenance was: lenalidomide based- 63%, bortezomib based- 11%, other- 1% and none- 24%.
Survival: Patients receiving VRD induction had superior outcomes compared to VCD induction with median PFS from transplant of 44.6 (38.0-55.8) months vs 34.1 (25.8-44.7) months, p 0.004 respectively. Median OS was not reached for either group, with 5-year OS 79 (74-83)% for VRD and 60 (50-69)% for VCD, p <0.001. Univariate PFS and OS outcomes by induction regimen and eGFR at diagnosis are shown in Table 2 and Figure 1. Multivariate analysis is shown in Table 3. After adjusting for prognostic factors, there was no statistically significant difference between the two induction regimens, with hazard ratio (HR) for PFS in the VCD vs VRD group being 1.22 (95% CI: 0.96-1.55, p=0.10). ISS stage, cytogenetics, disease status prior to transplant, and maintenance were independent prognostic factors for PFS. Similarly, on multivariate analysis for OS, there was no statistically significant difference in the two groups, with HR for OS in VCD vs VRD group being 1.33 (95% CI: 0.93-1.92, p=0.12). ISS stage, cytogenetics, disease status prior to transplant, and maintenance were independent prognostic factors for OS.
Conclusions: In patients with MM undergoing upfront transplant after VRD or VCD induction, no difference in outcomes was seen based on the induction therapy received after adjusting for other prognostic factors. As several MM patients with renal dysfunction are started on VCD, it is reassuring to see that VCD was not associated with inferior PFS, likely due to the eventual transition to maintenance therapy in the majority due to subsequent improvement of renal function. The use of maintenance treatment was uniformly associated with superior outcomes.
Sidana:Janssen: Consultancy. Kumar:Oncopeptides: Consultancy, Other: Independent Review Committee; IRC member; Kite Pharma: Consultancy, Research Funding; Genentech/Roche: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Celgene/BMS: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Dr. Reddy's Laboratories: Honoraria; Amgen: Consultancy, Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments, Research Funding; Janssen Oncology: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Takeda: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; AbbVie: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Novartis: Research Funding; Sanofi: Research Funding; MedImmune: Research Funding; Karyopharm: Consultancy; BMS: Consultancy, Research Funding; Tenebio: Other, Research Funding; Genecentrix: Consultancy; Carsgen: Other, Research Funding; Cellectar: Other; Merck: Consultancy, Research Funding; Adaptive Biotechnologies: Consultancy. Giralt:Johnson & Johnson: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Actinuum: Other: Advisory board, Research Funding; Amgen: Other: Advisory Board, Research Funding; KITE: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; OMEROS: Research Funding; SPECTRUM Pharma: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees, Research Funding; PFIZER: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jensenn: Membership on an entity's Board of Directors or advisory committees, Research Funding; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Research Funding; MILTENYI: Research Funding; JAZZ Pharmaceutical: Membership on an entity's Board of Directors or advisory committees. Qazilbash:Janssen: Research Funding; Bioclinica: Consultancy; Amgen: Research Funding; Angiocrine: Research Funding; Bioline: Research Funding. Shah:GSK, Amgen, Indapta Therapeutics, Sanofi, BMS, CareDx, Kite, Karyopharm: Consultancy; BMS, Janssen, Bluebird Bio, Sutro Biopharma, Teneobio, Poseida, Nektar: Research Funding. D'Souza:Janssen: Consultancy; Akcea: Consultancy; Imbrium: Consultancy; Merck: Research Funding; Pfizer: Consultancy; Teneobio: Research Funding; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal